ChemInform Abstract: A Novel [5 + 2] Cycloaddition Reaction Using a Dicobalt Acetylene Complex.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
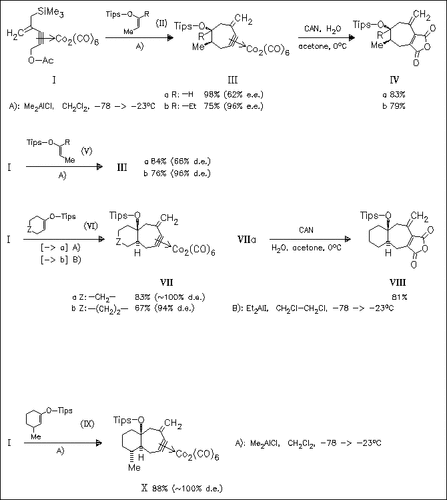
A novel five-carbon unit on the basis of a hexacarbonyldicobalt propargyl complex (I) is presented, which smoothly undergoes [5 + 2] cycloaddition reaction to various enol silyl ethers (II), (V), (VI) or (IX) to give methylenecycloheptane derivatives in good yields. It is shown that the adducts (III) are formed with high cis-selectivity irrespective of the geometry of starting silyl enol ether (II) or (V). In contrast, cyclic silyl enol ethers (VI) and (IX) react with excellent trans-selectivity. Additionally, even in the presence of a methyl group at the allylic position, the bicyclic adduct (X) is obtained with complete diastereofacial selection. Reaction of the hexacarbonyldicobalt complexes of the adducts with CAN does not regenerate the alkyne but provides the dioxofurano-fused analogues, e.g. (IV) and (VIII).