ChemInform Abstract: Asymmetric Aldol Reactions Using (S,S)-(+)-Pseudoephedrine-Based Amides: Stereoselective Synthesis of α-Methyl-β-hydroxy Acids, Esters, Ketones, and 1,3-syn and 1,3-anti Diols.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
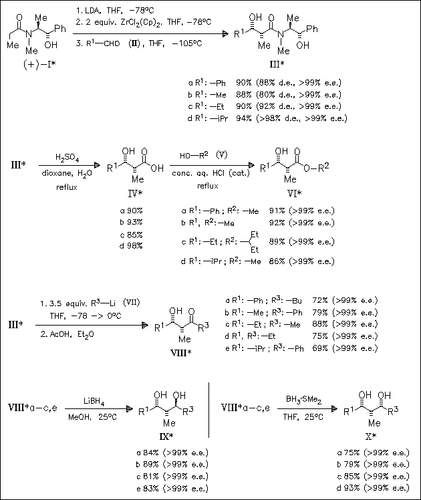
A very efficient method for performing highly stereoselective aldol reactions is developed. The resulting products can easily be transformed into α-methyl-β-hydroxy acids, esters, and ketones as well as 1,3-syn and 1,3-anti diols without racemization. The pheromones ent-sitophilure (VIIId) and ent-sitophilate (VIc) are available using this method.