ChemInform Abstract: The Chemistry of Indoles. Part 96. The First Total Synthesis of Bufobutanoic Acid by Two Routes Based on Nucleophilic Substitution Reaction on Indole Nucleus.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
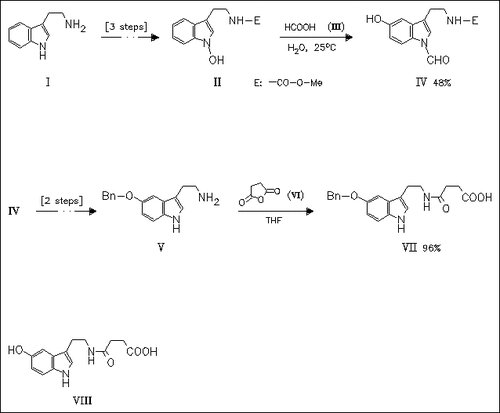
Two novel routes to the title bufobutanoic acid (VIII) are presented. The first route (25% overall yield) involves regioselective hydroxylation at position 5 of the N-hydroxyindole derivative (II). A sequence of protection—deprotection steps provides a synthetically useful benzyloxytryptamine (V), which on reaction with succinic anhydride provides benzyl-protected bufobutanoic acid (VII). In the second synthesis (13% overall yield) the carboxamido side chain is introduced first, followed by regioselective hydroxylation at the position 5.