ChemInform Abstract: Total Synthesis of Optically Active m-Phenylene PGl2 Derivative: Beraprost.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
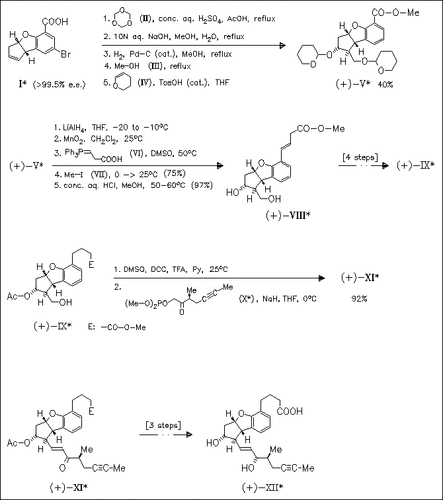
The synthesis of Beraprost as well as its diastereomers [demonstrated for one isomer (XII)] is described. Key steps of the synthesis are the introduction of side chains by Prins reaction, Wittig reaction, and Wadsworth reaction. By variation of the starting cyclopentanobenzofuran [(I) or its enantiomer] as well as variation of the Wadsworth reagent (X), four diastereomers of the title compound are accessible.