ChemInform Abstract: Transformation of 5,6,7,8-Tetrahydro-2H-1-benzopyran-2,8-diones with Hydrazines and Hydrazoic Acid: Synthesis of 8-Hydrazono-5,6,7,8-tetrahydro-2-oxo-2H-1-benzopyrans, Pyrano[2,3-c]azepines, and Pyrido[2,3-c]azepines.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
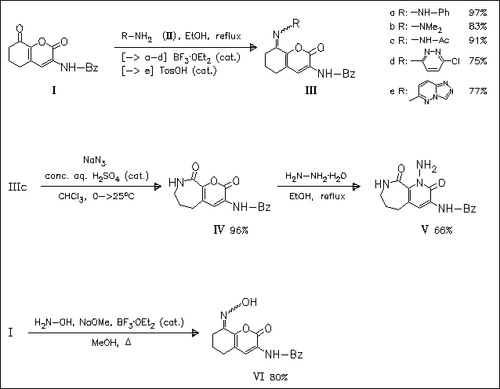
Reaction of tetrahydrobenzopyranylbenzamide (I) with N-nucleophiles, namely hydrazones and amines (II) or hydroxylamine, proceeds regioselectively at position 8 to furnish the corresponding imines (III) or hydroxyimine (VI), resp., in good yields. The expected reaction at position 2 and subsequent transformation of pyranone to pyridinone ring is not observed. Reaction of imines (III) with excess NaN3 (Schmidt reaction) proceeds by ring enlargement to provide access to pyrano-fused azepines like (IV). Additionally, reaction of compound (IV) with hydrazine or hydrazones affords pyridino-fused azepines like (V).