ChemInform Abstract: Preparation of 2-Substituted Naphth[2,3-d]oxazole-4,9-diones via a Radical Chain Process.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
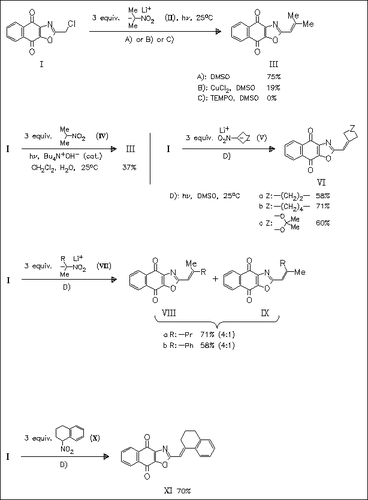
Reaction of chloromethylnaphthoxazoledione (I), prepared by bromination—halogen exchange reaction from methylnaphthoxazoledione, with nitroalkane anions proceeds via an SRN1 mechanism to provide access to ethylenic naphthoxazolediones (III), (VI), (VIII)/(IX) and (XI). The reaction mechanism is confirmed by use of radical chain inhibition experiments. Reaction with unsymmetrical nitroalkanes (VII) and (X) proceeds with high to excellent E-selectivity.