ChemInform Abstract: Synthesis of Bactericides via Carbon Nucleophilic Addition on 1,3-Diarylprop-2-enones as Michael Acceptors.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
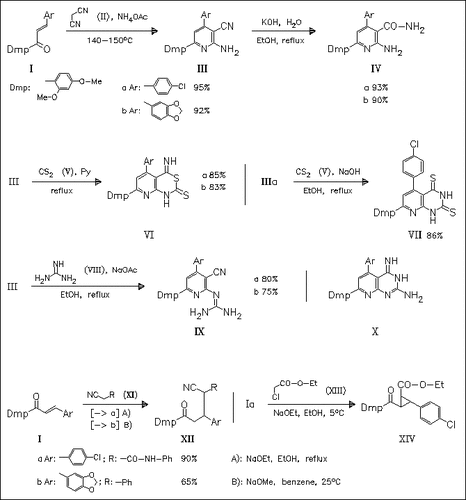
Michael addition reaction of chalcones (I) with malononitrile (II) followed by reaction with various N-nucleophiles provides a wide range of novel heterocyclic compounds. Similarly, chalcones (I) react with other C-nucleophiles like cyanomethylene compounds, chloroacetate, or benzaldehyde via a Michael addition pathway. Interestingly, the reaction of cyanoaminopyridine (III) with guanidine hydrochloride (VIII) does not afford the expected pyridinopyrimidines (X) but the open-chain isomers (IX). Among the novel heterocyclic compounds tested, derivatives (IVb) and (IXb) possess promising activity against Gram-positive and Gram-negative bacteria.