ChemInform Abstract: Unusual Ring Contraction by Substitution of 4-O-Activated-pentono-1,5-lactams with Cyanide. Stereospecific Synthesis of 6-Amino-1,4,5,6- tetradeoxy-1,4-imino-hexitols.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
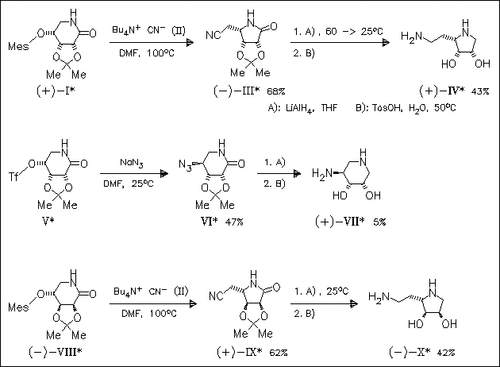
Reaction of 4-O-sulfonylated D-ribo- (I) or D-lyxo-1,5-lactams (VIII) with Bu4N+CN- proceeds by ring contraction with inversion of the configuration at position 4 to furnish C-5-cyanated derivatives (III) and (IX), respectively. Reduction of these derivatives provides the corresponding amino-hexitols (IV) and (X), resp., which are screened for their inhibiting activity on α-L-fucosidase. Interestingly, this stereospecific ring contraction proceeds only during cyanation reaction. Reaction of sulfonylated ribolactam (V) with NaN3 affords the expected 4-azidolactam (VI).