ChemInform Abstract: Design of Novel Derivatives of Phosphonoformate (Foscarnet) as Prodrugs and Antiviral Agents.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
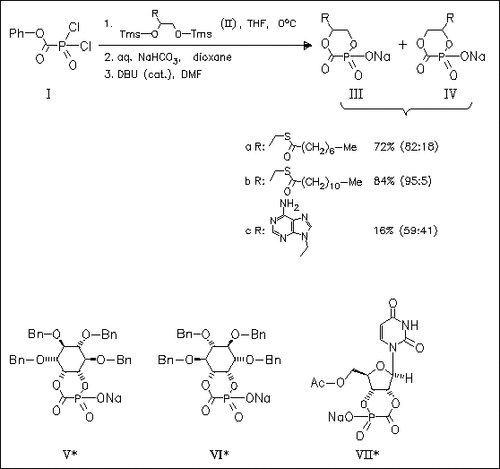
Antiviral phosphonoformate (PFA) is an effective agent against AIDS and HIV itself, but possesses poor bioavailability. Thus, novel cyclic PFA diesters (III)-(VII) are synthesized as potential prodrugs and antiviral agents, in which the PFA moiety is incorporated within biomimetics of nucleotides, carbohydrates, and phospholipids. As described below, the in situ synthesis is achieved following a published procedure, but avoids the required isolation of the acyclic precursor and leads to a mixture of regioisomers (III)/(IV). Carbohydrate derivatives (V) and (VI) are prepared analogically and uridine derivative (VII) is isolated as single isomer. In antiviral assays on HSV-1-infected confluent human fibroblast cells diester (IIIb) shows comparable activity to PFA itself whereas the activity of derivatives (IIa), (IIIc), and (VII) is somewhat less.