ChemInform Abstract: Wittig Rearrangement of Allyl and Propargyl Furfuryl Ethers Leading to 2-Furylmethanol Derivatives.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
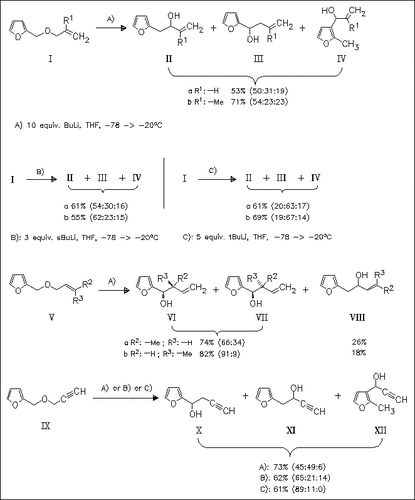
The first example of the Wittig rearrangement of allyl and propargyl furfuryl ethers like (I), (V), and (IX) is given providing a variety of furyl alcohols, especially 2-furylmethanol derivatives. To gain an understanding of the stabilities of the deprotonated educts, ab initio calculations are undertaken. The diastereoselectivity in the rearrangement of (V) is influenced by the solvent used. Furthermore, the application of the new route to the preparation of 3-(2-furyl)-3-hydroxy-2-methylpropionates is given.