ChemInform Abstract: Scope and Limitations of the Pd/BINAP-Catalyzed Amination of Aryl Bromides.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
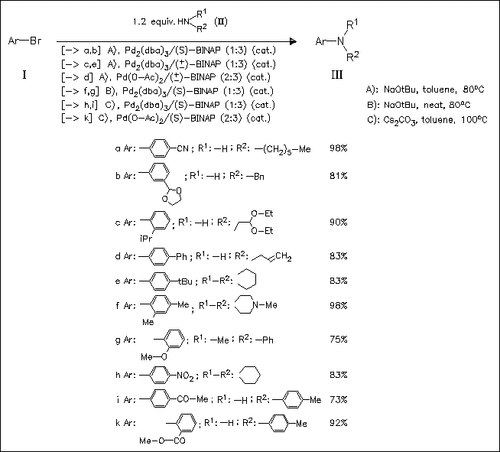
The Pd/BINAP catalyst system is highly effective for the arylation of primary amines. A variety of substrate combinations are tolerated, including amines that are branched or contain functional groups such as olefins or acetals. Good results can also be obtained for arylation of secondary amines, although acyclic secondary amines often react poorly under these conditions. A wide variety of functional groups such as ester, nitrile, nitro groups, and enolizable ketones are tolerated when the mild base Cs2CO3 is employed.