ChemInform Abstract: Use of Metalated Allylic Ethers for the Elaboration of Vicinally Trisubstituted Linear Substrates or Cyclopropyl Carbinols.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
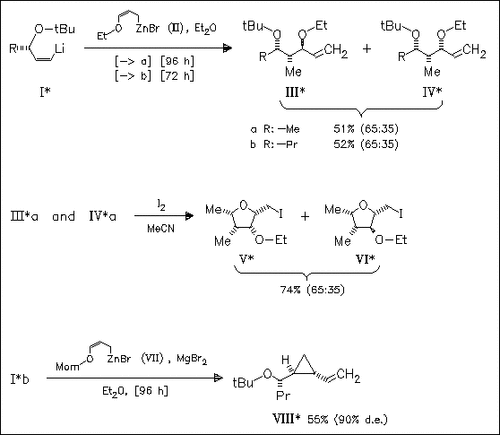
The carbometalation of secondary allylic ethers (I) by allylic zinc reagents is investigated. Thus, the reaction of (I) and (II) in ether leads to a mixture of diastereomers (III) and (IV) which is converted into ethers (V) and (VI) by iodoetherification. However, the MOM ether (VII) gives with (Ib) in ether by treatment with MgBr2 diastereoselectively the trans cyclopropyl carbinol (VIII).