ChemInform Abstract: A Versatile One-Pot Synthesis of 1,3-Substituted Guanidines from Carbamoyl Isothiocyanates.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
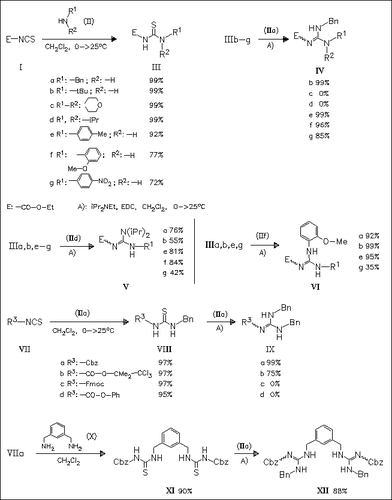
Carbamoyl isothiocyanates are ideal starting materials for the two-step one-pot synthesis of multisubstituted guanidines from two separate amines. In the first step, carbamates of type (I) or (VII) are coupled with aliphatic or aromatic amines to give thioureas in excellent yields which are coupled in the second step with another amine to afford the desired guanidines. Thioureas (IIIc) and (IIId) as well as (VIIIc) and (VIIId) failed to form guanidines in detectable yields. Multisubstituted guanidine (XII) is also obtained by this method.