ChemInform Abstract: Replacing Tin in Radical Chemistry: N-Ethylpiperidine Hypophosphite in Cyclization Reactions of Aryl Radicals.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
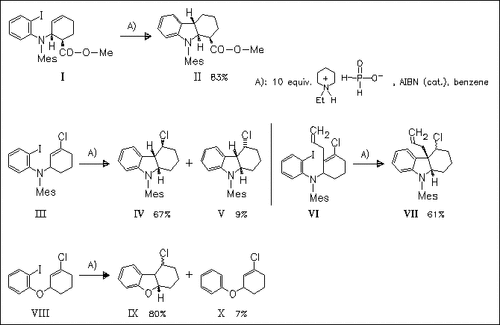
The use of N-ethylpiperidine hypophosphite (EPHP) in radical cyclization reactions shows that the reagent has some advantages over tributyltin hydride. Thus, the cyclization proceeds well and with respectable yields, even when the substrates present terminal alkenes. Furthermore, the phosphorus-centered radicals show higher chemoselectivity over tributyltin radicals (syringe-pump addition of EPHP can improve the yields).