ChemInform Abstract: Highly Regio- and Stereoselective Cycloreductions of 1,6- and 1,7-Enynes Activated with a Carbonyl Functionality.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
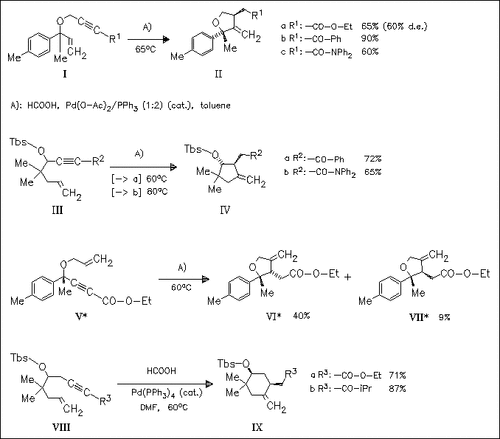
A highly regio- and stereoselective cycloreduction of 1,6- and 1,7-enynes, in which the alkyne unit is conjugated with an electron withdrawing group, to γ,δ-unsaturated compounds is reported. Mechanistically, a two-step β-elimination—reduction sequence is assumed.