ChemInform Abstract: Ruthenium-Catalyzed Intramolecular [5 + 2] Cycloadditions.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
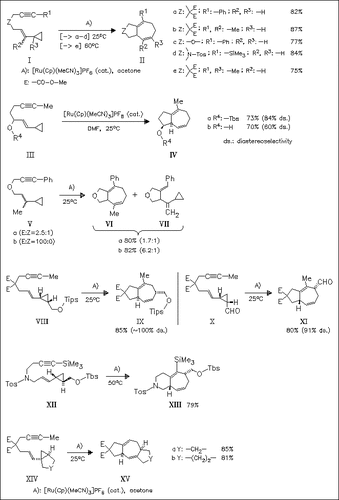
Intramolecular [5 + 2] cycloaddition reaction of vinylcyclopropanes tethered to an alkyne is efficiently catalyzed by the cationic ruthenium complex [Ru(Cp)(MeCN)3]PF6 in acetone. A wide range of substrates is tolerated, providing cyclopentane-, pyrrolidine-, tetrahydrofuran- or piperidine-fused cycloheptadiene derivatives in good yields. In the case of compounds (V), mixtures of a cycloadduct (VI) and a product of β-hydride elimination (VII) are obtained. It is shown that the cycloadduct (VI) mainly arises from E-isomer (Vb). The diastereoselectivity of cycloaddition is studied by varying the substituents at the double bond as well as on the cyclopropyl residue. More complicated ring systems (XV) are obtained in complete regio- and diastereoselectivity using this methodology.