ChemInform Abstract: Synthesis of a New Chiral Oxazolidinone Auxiliary Based on D-Xylose and Its Application to the Staudinger Reaction.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
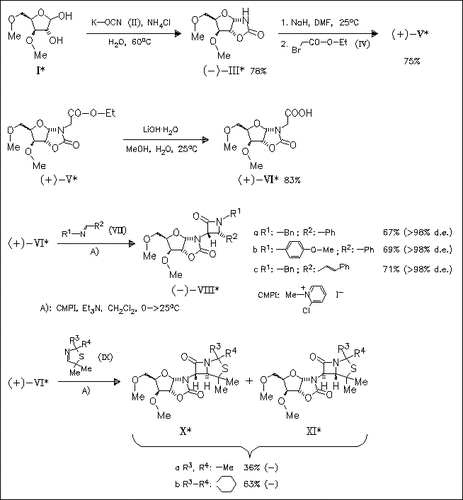
Reaction of partially protected D-xylose (I) with KOCN provides a novel chiral oxazolidinone derivative (III), which is used as chiral auxiliary in the diastereoselective Staudinger-type β-lactam formation from ketenes and imines. Thus, reaction of the auxiliary-bound acetic acid (VI) with several imines (VII) in the presence of N-methyl chloropyridinium iodide as dehydrating agent affords β-lactams (VIII) in good yields and excellent cis-selectivity. As expected, the analogous reaction with cyclic imines (IX) proceeds with excellent trans-selectivity to give trans-isopenam derivatives (X)/(XI).