ChemInform Abstract: Glycosylation-induced Asymmetric Synthesis: -Amino Acid Esters via Mannich Reactions.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
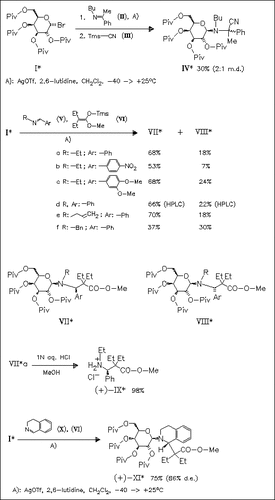
Schiff bases (II), (V) and (X) are smoothly activated by N-glycosylation reaction with glycosyl bromide (I) to stereoselectively undergo Mannich reaction with silyl ketene acetals providing the β-amino esters in good yields and moderate to good diastereoselectivity. Diastereoselectivity is decreased, when the intermediate iminium ion can undergo transformation to enamine, or when large N-substituents [cf. (Vf)] hinder the E→Z isomerization of the C—N double bond in iminium intermediate.