ChemInform Abstract: The Oxetane Ring in Taxol.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
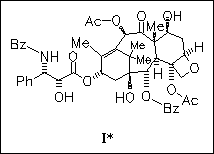
The oxetane ring of anticancer drug taxol (I) is regarded to be essential for the biological activity of the title compound. Computer-aided investigation of the structure—activity relationship ascertains the fact that it is not necessary, at least as it pertains to microtubule assembly, as supported by studies of oxetane heteroatom replacement, transformation of the oxetane into a three-membered ring, and transformation of taxol into D- or C-seco analogues. Thus, further optimization is of interest and will lead to the design and development of novel anticancer drugs.