ChemInform Abstract: Cyclic (4S)-Chloromethyl Sulfite and Sulfate Derivatives of (S)-Glycidol as Valuable Synthetic Equivalents of Scalemic Epichlorohydrin.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
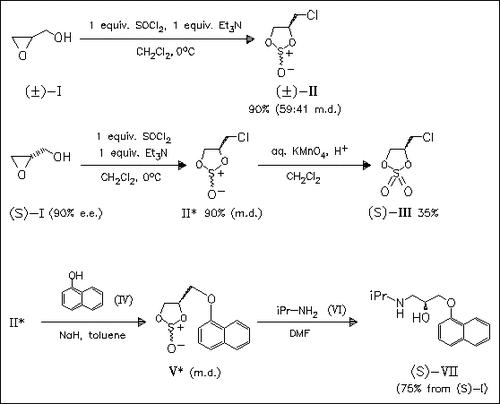
The interaction of (S)-glycidol (I) with SOCl2 or SO2Cl2 in stoichiometric amounts leads to the formation of cyclic (4S)-chloromethyl sulfite (II) or sulfate (III) with comparable enantiomeric purity. Based on this reaction, a new procedure for the synthesis of the β-adrenoreceptor blocker (S)-propanolol (VII) is developed.