ChemInform Abstract: Stereoselection in the Prins-Pinacol Synthesis of 2,2-Disubstituted 4-Acyltetrahydrofurans. Enantioselective Synthesis of (-)-Citreoviral (XII).
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
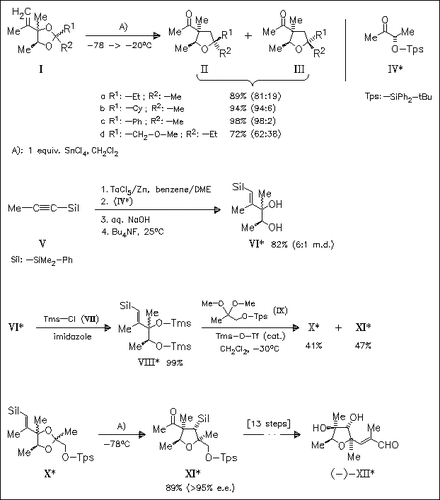
The condensation of allylic diols with unsymmetrical ketones [cf. (VIII) and (IX)] proceeds with high stereoselectivity when the α-substituents of the ketone differ substantially in size. Rearrangement of the ketal thus obtained according to A) allows a selective formation of the desired furan fragment.