ChemInform Abstract: Asymmetric Synthesis of α-Amino Acids Based on Carbon Radical Addition to Glyoxylic Oxime Ether.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
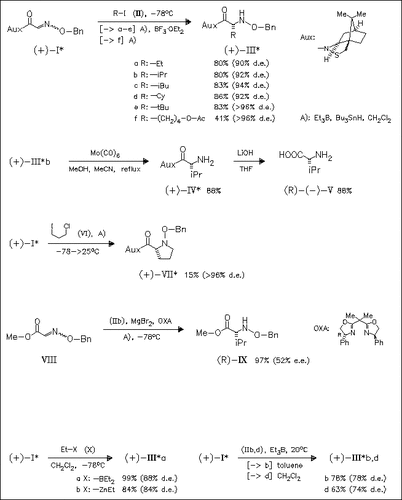
The new carbon radical addition to oxime ethers (I) and (VIII) is studied by varying the reaction conditions such as the presence or absence of Bu3SnH, the type of Lewis acid (e.g. BF3×OEt2 or MgBr2), the type of initiator (Et3B or Et2Zn), the applied solvent, and reaction temperature. A range of enantiomerically pure natural and unnatural α-amino acids may be derived by this method after reductive removal of the benzyloxy and the auxiliary groups.