ChemInform Abstract: Bis(phospholane) Ligands Containing Chiral Backbones. Matching and Mismatching Effects in Enantioselective Hydrogenation of α-Keto Esters.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
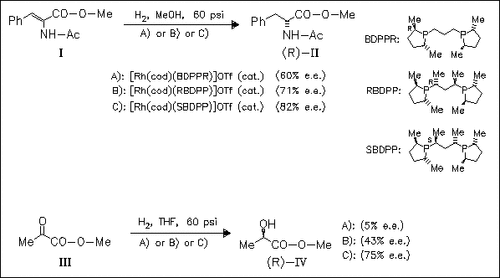
The synthesis of several novel bis(phospholane) ligands containing a chiral 2,4-pentylene backbone, e.g. SBDPP and RBDPP, is described. Their utility is studied in the hydrogenation of α-aminocarboxylic esters like (I) and of α-keto ester (III) in the presence of a [Rh(cod)(ligand)]OTf catalyst. It is shown, that the sense of stereoinduction is determined by the phospholane stereochemistry of the ligand, since reduction products of the same configuration are obtained irrespective of the configuration of the ligand backbone. In contrast, the degree of stereoinduction strongly depends on the nature of ligand backbone. The best results are obtained for ligand SBDPP (75% e.e.), while with diastereomeric ligand (RBDPP) as well as the parent compound (BDPPR) the reduction products are obtained in only low optical purity.