ChemInform Abstract: Synthesis of 4-Oxo-2-alkenylphosphonates via Nitrile Oxide Cycloaddition: γ-Acylation of Allylic Phosphonates.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
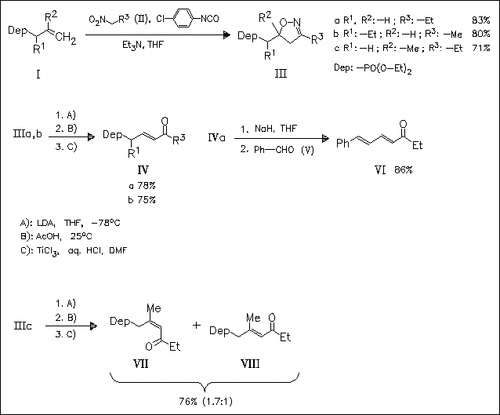
A new method for the regioselective γ-acylation of allylic phosphonates is presented involving the cycloaddition of in situ generated nitrile oxides to allylic phosphonates, followed by eliminative ring fission on treatment with LDA and AcOH, and final hydrolysis by aqueous HCl solution of TiCl3. Products bearing no substituent in the β-position are exclusively obtained as E isomers, whereas the Z isomer dominates for β-substituted compounds such as (VII). The utility of the oxophosphonates as building blocks for the synthesis of polyethylene-structured natural compounds is demonstrated with the synthesis of diene (VI).