ChemInform Abstract: A New Diastereoselective Synthesis of a 1β-Methylcarbapenem Intermediate.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
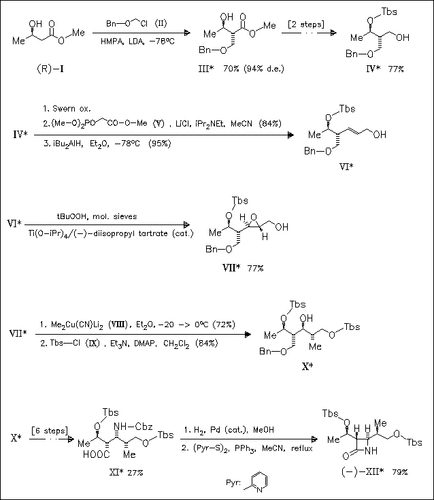
A new method for the synthesis of 1β-methylcarbapenem key intermediate (XII) starting from commercially available methyl (R)-3-hydroxybutyrate (I) is given. The key features in this strategy are the diastereoselective alkylation of the dianion of the β-hydroxy ester (I), Sharpless asymmetric epoxidation, and subsequent regioselective cuprate ring opening of the chiral epoxide (VIII) formed.