ChemInform Abstract: Facile Synthesis and Conformation of 3′-O,4′-C-Methyleneribonucleosides.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
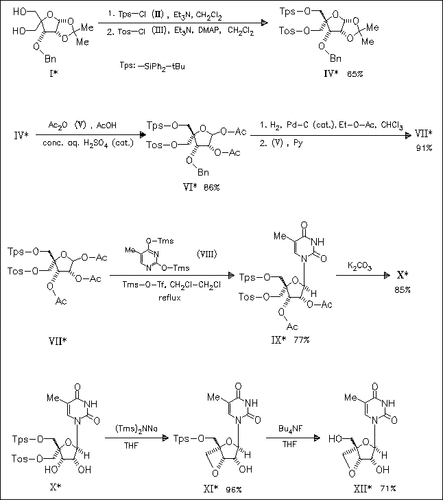
A novel and practical route to the title compounds (XII) (4 examples) involving the coupling of 1-O-acetylribofuranose derivatives [cf. (VII)] with silylated nucleobase [→(IX)] and a subsequent oxetane ring formation [→(XI)] as the key steps is developed. NMR and X-ray analysis indicates that the ribofuranose ring of nucleoside analogue (XII) is found to exist predominantly in a S-conformation.