ChemInform Abstract: Enzymes in Organic Synthesis. Part 15. Short Enzymatic Synthesis of L-Fucose Analogues.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
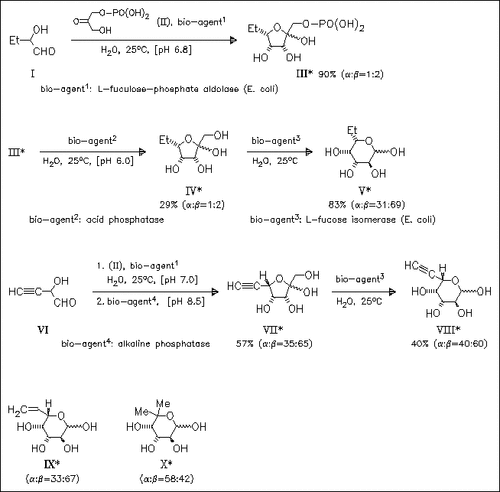
A short and efficient enzymatic route to L-fucose analogues substituted with hydrophobic groups is described. Cyclocondensation of in situ generated α-hydroxyaldehydes [cf. (I), (VI)] with dihydroxyacetone phosphate (II) proceeds with absolute diastereoselectivity in the presence of L-fuculose 1-phosphate aldolase to furnish furanose phosphates like (III), which are smoothly dephosphorylated with the appropriate phosphatase. Isomerization of the so obtained ketose [cf. (IV), (VII)] to aldose derivatives in the presence of L-fucose isomerase is decelerated with increasing steric bulk of substrate, so that compound (X) is obtained in 34% yield only.