ChemInform Abstract: Umpolung of Carbon—Sulfur Bonds. Novel Synthesis of Substituted Allenes from Propargylic Dithioacetals.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
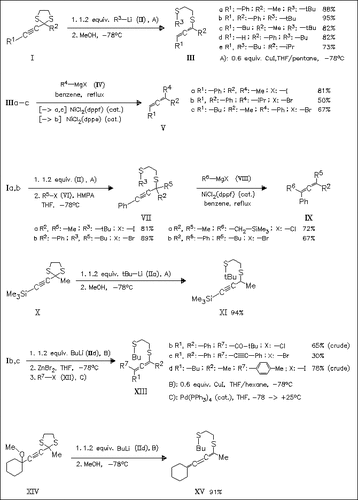
Treatment of propargylic dithioacetals like (I) with organocuprates followed by protonolysis generally leads to allenyl thioethers of type (III). In the presence of alkyl halides propargylic thioethers such as (VII) are formed. Transmetalation of organocopper intermediates with ZnBr2 and subsequent Pd-catalyzed coupling with alkyl halides leads to allenyl thioethers. Thioethers of type (III) and (VII) react with Grignard reagents to afford allenes like (V) and (IX). Thus, using these procedures di-, tri-, and tetrasubstituted allenes can be prepared conveniently. Additionally, it is shown that the cumulene (XV) can also be synthesized under these conditions. However, the formation of higher cumulenes is unsuccessful.