ChemInform Abstract: Hydroxylation and Amination of Azulenes by Vicarious Nucleophilic Substitution of Hydrogen.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
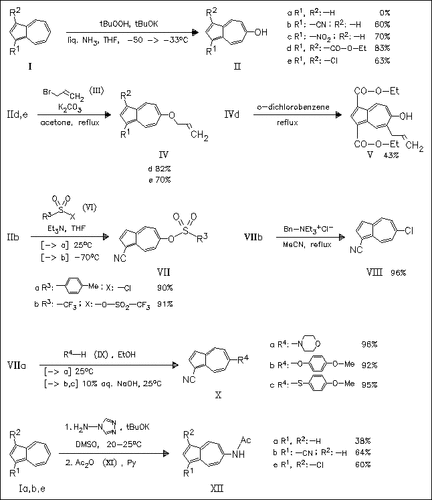
Vicarious nucleophilic substitution is shown to be a versatile tool for the introduction of several O-, N-, or S-substituents into the azulene skeleton. Hydroxylation of azulene derivatives (I) with tert-butylhydroperoxide proceeds smoothly in the presence of electron-withdrawing substituents to furnish the 6-hydroxyazulenes (II) with absolute regioselectivity. These hydroxy derivatives can be used to introduce, via several sulfonyloxy analogues (VII), nucleophiles into position 6 [cf. (VIII) and (X)]. Additionally, allylation of 6-hydroxy derivatives (II) in the presence of K2CO3 affords the corresponding O-allyl derivatives (IV), which readily undergo Claisen rearrangement to provide access to C-allylated azulenes. Amination of azulenes (I) also proceeds smoothly and regiospecifically even in the case of unsubstituted azulene (Ia).