ChemInform Abstract: The Stille Reaction of 1,1-Dibromo-1-alkenes: Preparation of Trisubstituted Alkenes and Internal Alkynes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
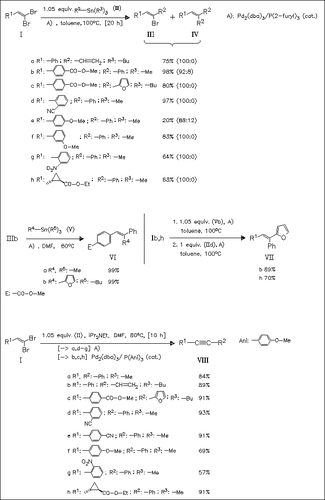
Stille coupling of dibromoalkenes of type (I) with organostannanes leads to (Z)-monobromides like (III) in good to high yields when the reaction is run in low to nonpolar solvents with the ligand P(o-Fur)3. This coupling is less suitable for 2-aryl-1,1-dibromoalkenes bearing strongly electron donating substituents at ortho or para positions. It can be applied to the stereospecific synthesis of trisubstituted alkenes. Stille coupling of (I) in a higher polar solvent gives internal alkynes like (VIII) in high yields. This represents a new, general, and mild approach to internal alkynes.