ChemInform Abstract: Regio- and Stereoselective Oxidative Difunctionalization of Alkylidene Cyclohexenes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
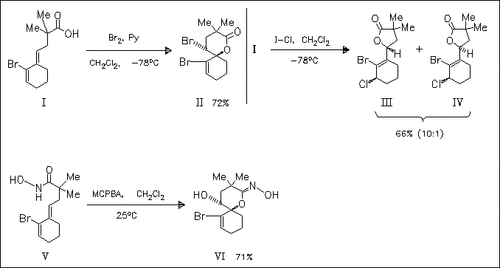
Oxidation of the alkylidene cyclohexene (I) with bromine affords a spirocyclic δ-lactone whereas treatment with I-Cl leads to the γ-lactones (III) and (IV). Interestingly, epoxidation of the related hydroxamic acid (V) results in formation of the δ-lactone oxime (VI).