ChemInform Abstract: (-)-Sparteine-Mediated Stereoselective Intramolecular Carbolithiation of 4-Substituted 5-Hexynyl Carbamates. Synthesis of Enantiopure 1,3-Difunctionalized Alkylidene Cyclopentanes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
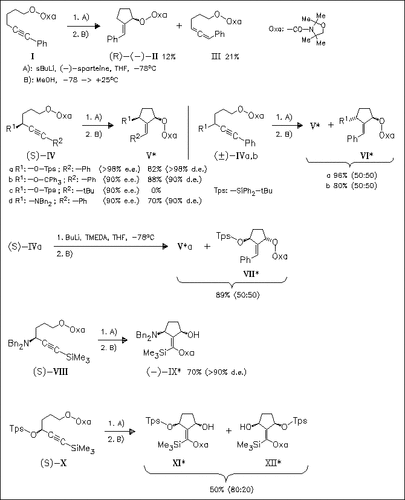
Treatment of 5-hexynyl carbamates with sBuLi/(-)-sparteine offers a novel method for the stereoselective synthesis of functionalized alkylidene cyclopentanes. The reaction outcome strongly depends on the substitution pattern of the educt. Thus, starting from the 4-substituted carbamates (IVa), (IVb), and (IVd) good results are obtained, whereas the tBu-substituted analogue (IVc) and 4,4-disubstituted derivatives does not yield cyclopentanes. The 4-unsubstituted carbamate (I) gives the cyclopentane (II) in poor yields. Interestingly, starting from the Me3Si-substituted derivatives (VIII) and (X) migration of the carbamoyl group takes place. This method cannot be used for the kinetic resolution of alkynes as shown by the reaction of racemic (IV). Additionally, it is found that the propargylic stereocenter has no effect on the stereochemical outcome of deprotonation.