ChemInform Abstract: Coupling Reactions. Part 21. Synthesis of Functionalized Bi(cyclopropylidenes).
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
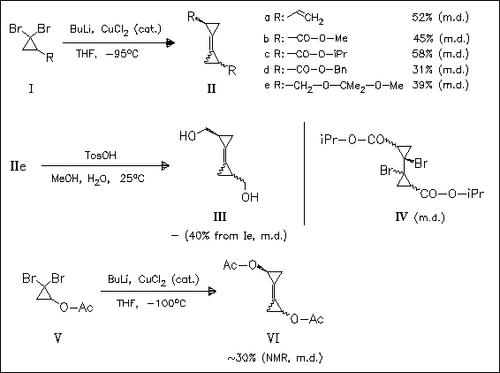
In the search for novel triafulvalene precursors, a series of bi(cyclopropylidenes) (II) is prepared by copper-catalyzed dimerization of the corresponding dibromocyclopropane derivatives (I). However, the synthesis of carboxy-substituted bi(cyclopropylidene), a suitable precursor for dibromo-bi(cyclopropylidene), is not achieved, neither by direct dimerization reaction nor by hydrolysis of alkoxycarbonyl analogues (IIb)—(IId) nor by oxidation of hydroxymethyl analogue (III). Finally, direct synthesis of acetoxy-substituted bi(cyclopropylidene) (VI), bearing suitable leaving groups for triafulvalene formation, is achieved, although in low yield.