ChemInform Abstract: The Cluster Compounds Ag[W6Br14] and Ag2[W6Br14].
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
The reaction of W6Br12 with AgBr in a temperature gradient (925→915 K; evacuated silica tube) yields brownish black octahedral crystals of Ag[W6Br14] (I) and yellow-green platelets of Ag2[W6Br14] (II). (I) crystallizes in the cubic space group Pn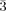 (Z = 4) and is isotypic with Hg[Mo6Cl14]. (II) has a monoclinic structure, space group P21/c (Z = 2). Both structures contain isolated [W6Br14]1-/2- cluster anions consisting of an octahedral W6 core, 6 terminal Br ligands, and 8 face-capping Br ligands. The Ag+ cations in (I) are trigonal antiprismatically coordinated and those in (II) have a distorted trigonal-planar coordination sphere.
(Z = 4) and is isotypic with Hg[Mo6Cl14]. (II) has a monoclinic structure, space group P21/c (Z = 2). Both structures contain isolated [W6Br14]1-/2- cluster anions consisting of an octahedral W6 core, 6 terminal Br ligands, and 8 face-capping Br ligands. The Ag+ cations in (I) are trigonal antiprismatically coordinated and those in (II) have a distorted trigonal-planar coordination sphere.




