ChemInform Abstract: Synthesis of New Chiral 18-Crown-6 Ethers from D-Xylose.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
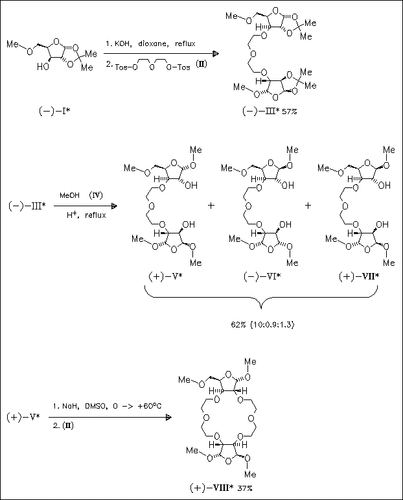
A facile and efficient synthesis of chiral 18-crown-6 ethers containing two furanoside moieties is presented. Compound (I), obtained from D-xylose, undergoes ether formation at the free hydroxyl group to give ethyleneoxyethyl bridged carbohydrate (III). Methanolysis of the isopropylidene protecting group of compound (III) affords a separable mixture of diastereomeric dihydroxides (V)-(VII), each suitable for ring closing reaction with diethylene glycol ditosylate (II). This method provides access to a wide variety of symmetrical or unsymmetrical chiral crown ethers containing two furanoside moieties.