ChemInform Abstract: Nickel-Catalyzed Carbostannylation of Alkynes with Allyl-, Acyl-, and Alkynylstannanes: Stereoselective Synthesis of Trisubstituted Vinylstannanes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
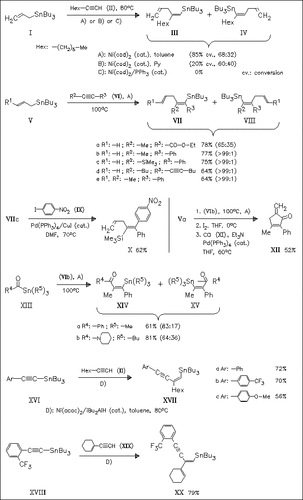
The addition of alkynyl- (XVI), (XVIII), allyl- (I), (V), and acylstannanes (XIII) to both electron-deficient and electron-rich alkynes (II), (VI) is achieved in the presence of nickel catalysts. The reaction proceeds with high regio- and syn-selectivity to afford differently substituted alkenylstannanes in moderate to good yields. The synthetic utility of the obtained alkenylstannanes is demonstrated. Thus, they are suitable educts in the Heck reaction with aryl iodides, as shown for the formation of compound (X). Additionally, organylstannane (VIIb) can undergo, after iodination, palladium-catalyzed cyclo-carbonylation to afford methylenecyclopentenone derivative (XII).