ChemInform Abstract: Spiro-imidazo[1,2-a]indeno[1,2-e]pyrazine-4-one Derivatives are Mixed AMPA and NMDA Glycine-Site Antagonists Active in vivo.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
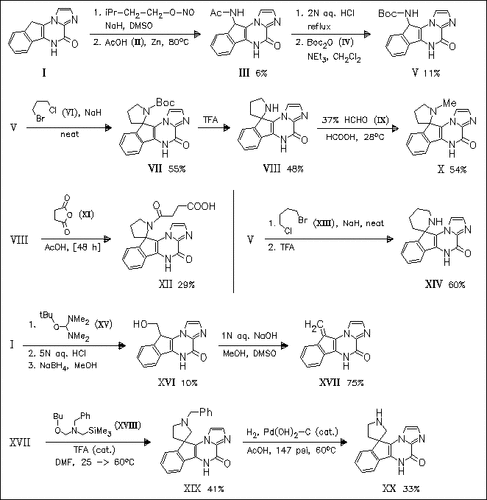
Several spiro-imidazo-indeno-pyrazinone derivatives like (X), (XII), (XIV), and (XX) are synthesized and tested for their biological activity. Key steps of the synthesis are the preparation of key amino intermediate (III) and the regioselective [3 + 2] cycloaddition reaction of (XVII) with (XVIII), respectively. The new compounds exhibit good affinities for both AMPA and the NMDA glycine site receptors and display good anticonvulsant effects. The racemic compound (X) is separated into both enantiomers by chromatography. It is found that the corresponding dextrorotatory isomer (+)-(X) is notably more potent than the levorotatory isomer (-)-(X).