ChemInform Abstract: Efficient Tin Hydride-Mediated Radical Cyclization of Secondary Amides. Part 1. Synthesis of a Variety of Substituted Pyrrolidinones.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
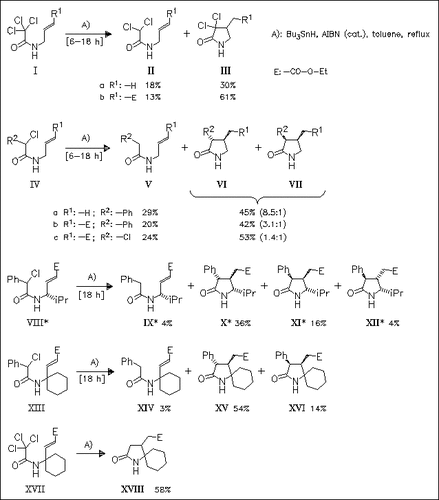
Secondary haloamides are demonstrated to undergo free radical cyclization reactions in boiling toluene. Bulky N-protection groups, established for related cyclizations, are not required. Diastereoselectivity and efficiency of these reactions are found to depend on nature and position of the C-chain substituents.