ChemInform Abstract: Bonded peri-Interactions Govern the Rate of Racemization of Atropisomeric 8-Substituted 1-Naphthamides.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
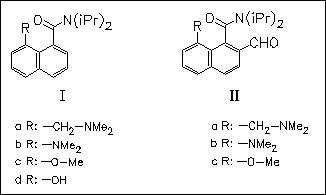
8-Substituted tertiary 1-naphthamides (I), (II) are chiral, atropisomeric compounds at room temperature, and the rate at which they racemize depends on the extent to which the 8-substituent undergoes incipient nucleophilic addition to the amide carbonyl group, as indicated by X-ray crystallography.