ChemInform Abstract: Reaction of Sterically Congested Thiones with Benzyne. The First Isolation of a Thioaldehyde—Benzyne Adduct.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
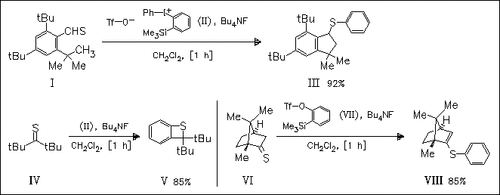
Sterically congested aromatic thioaldehyde (I) reacts with benzyne, in situ generated from iodonium compound (II), to give a 1:1 thioaldehyde—benzyne adduct (III), the structure of which is confirmed by spectroscopic means. A radical mechanism is proposed for this synthesis. In contrast, normal cycloaddition of sterically congested aliphatic thioketone (IV) with benzyne leads to the [2 + 2] cycloadduct (V), whereas reaction of sterically less hindered thiocamphor (VI) with benzyne precursor (VII) leads to ene-reaction product (VIII).