ChemInform Abstract: Highly cis- and Enantioface-Selective Cyclopropanation Using (R,R)-Ru—Salen Complex: Solubility Dependent Enantioface Selection.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
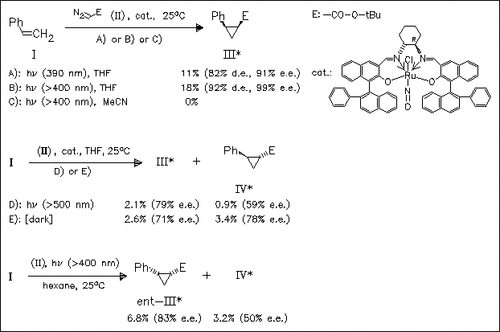
Cyclopropanation of styrene (I) with diazoacetate (II) in the presence of a chiral ruthenium—salen complex under irradiation with incandescent light in THF provides the (1S,2R)-phenylcyclopropanecarboxylate (III) in high diastereo- and enantioselectivity. In contrast, the same reaction in hexane affords the corresponding (1R,2S)-enantiomer (ent-III) in slightly lower stereoselectivity. It is proposed that in solvents like THF or DME, which dissolve the catalyst, a monomeric activated catalytic species is formed, while in hexane or iPr2O the reaction takes place at the surface of insoluble catalyst, involving a different ligand conformation.