ChemInform Abstract: Photochemistry of Fluorophenyl Azides in Diethylamine. Nitrene Reaction versus Ring Expansion.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
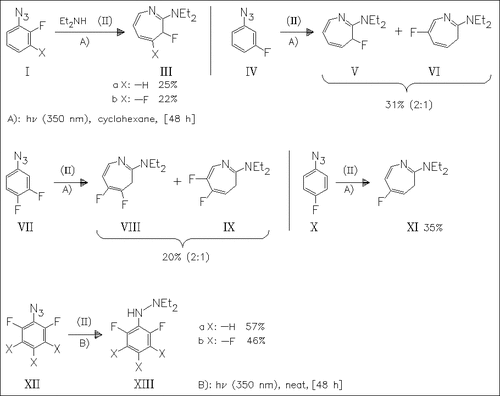
Fluorophenyl azides are the most popular reagents for photoaffinity labeling. In order to further investigate the fluorine effect on the singlet nitrene ring expansion, several fluorophenyl azides were photolyzed with diethylamine at 25 °C. While most of the compounds afford azepines by ring expansion, in o,o′-difluorosystems such as (XII) a steric repulsion leads to a considerably higher barrier to ring expansion producing hydrazines by singlet phenyl nitrene N—H insertion reactions.