ChemInform Abstract: Bicyclo[3.3.0]octanone Formation by Fe(III) Mediated Ring Expansion-Transannular Cyclization Reactions of Cyclopropyl Ethers.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
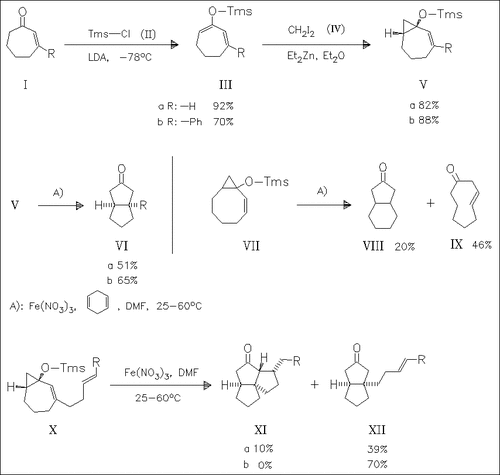
Readily prepared bicyclo[5.1.0]octenes (V) undergo a Fe(III)-mediated ring expansion-transannular cyclization reaction to yield bicyclo[3.3.0]octanones (VI). The corresponding bicyclo[6.1.0]nonene (VII) gives under similar conditions mainly the nonenone (IX). Interestingly, the transannular cyclized radical can be intermolecularly intercepted by a suitably placed alkene chain. However, yields of the resulting tricycles (XI) are low.