ChemInform Abstract: Total Synthesis of Macrolactin A with Versatile Catalytic, Enantioselective Dienolate Aldol Addition Reactions.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
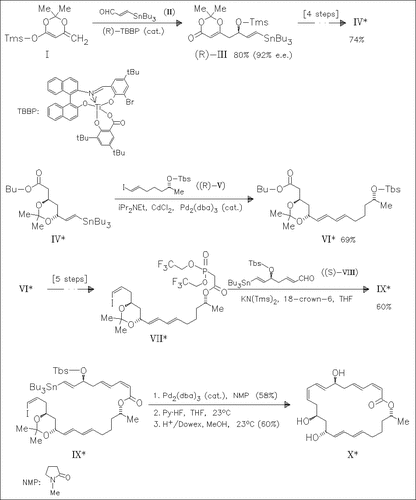
Two of three key subunits of macrolactin A (X) containing the desired stereogenic centers are prepared via the title reaction starting from the same precursors (I) and (II) in the presence of either the (R)- or (S)-enantiomeric form of the titanium(IV) catalyst, leading to both enantiomers of (III).The latter are elaborated to give fragments (VII) and (VIII), respectively. Palladium(0)-catalyzed Stille coupling is used to assemble the principal fragments.