ChemInform Abstract: A β-Lactone-Based Strategy Applied to the Total Synthesis of (8S,21S,22S,23R)- and (8R,21S,22S,23R)-Okinonellin B.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
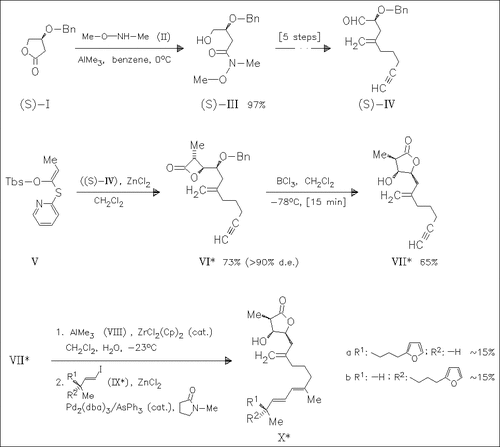
The key steps in the synthesis of the title compounds (X) are tandem Mukaiyama aldol—lactonization of the aldehyde (IV) and tandem debenzylation—transacylation of the β-lactone (VI). Natural okinonellin B presumably possesses (8R,21R,22R,23S) stereochemistry.