ChemInform Abstract: Rearrangement of an N-Aryl-2-vinyltetrahydro-4-oxoquinoline to an Acridine Derivative.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
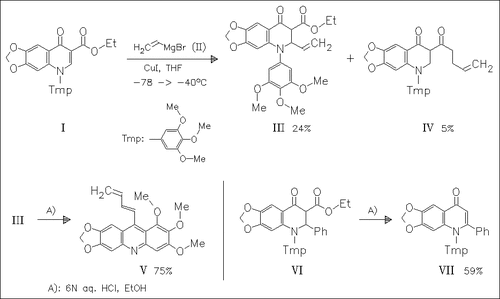
Acidic treatment of the readily prepared quinoline (III) results in rearrangement to the acridine (V) via a retro-Michael process and a following attack of the electron-rich aromatic ring onto the keto group. Interestingly similar educts, e.g. the phenyl derivative (VI) fail to give this rearrangement. The reasons for the differences are not clear.