ChemInform Abstract: An Efficient and Stereoselective Conversion of Lactones to Substituted Cyclic Ethers.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
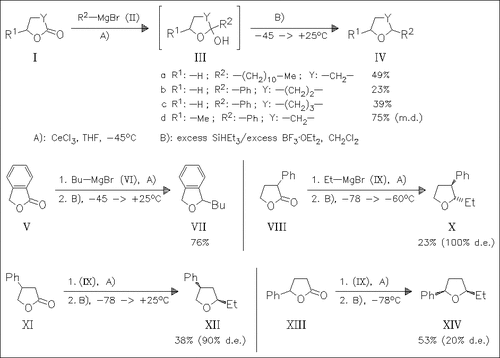
Cerium(III) chloride assisted addition of Grignard reagents to lactones and following BF3×OEt2 mediated deoxygenation of the resulting hemiketals [cf. (III)] with triethylsilane provides a general route to cyclic ethers. The process is also effective for the diastereoselective formation of cyclic ethers derived from monosubstituted tetrahydrofuranones.