ChemInform Abstract: Enantioselective Total Synthesis of the (-)-(6R,11R,14S)-Isomer of Colletallol.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
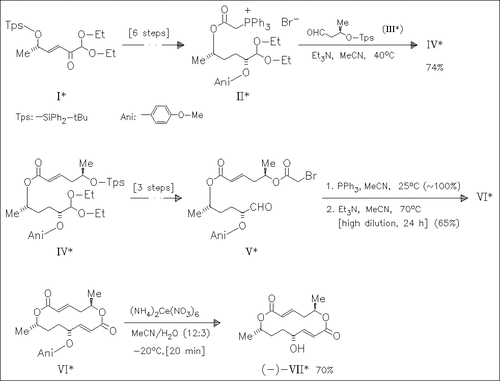
The total synthesis of title compound (VII) is based on two consecutive Wittig reactions, the first one intermolecular and the second one intramolecular, instead of classical macrolactonizations. The strategy is flexible and should be useful for the preparation of further macrolide antibiotics.