ChemInform Abstract: Synthesis and Receptor Binding Affinity of Conformationally Restricted Retinoic Acid Analogues.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
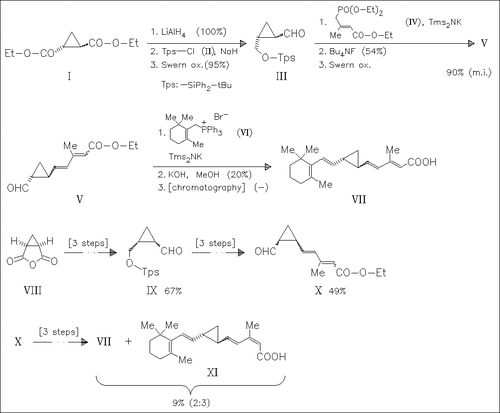
Efforts to synthesize two conformationally restricted retinoids utilizing a cyclopropyl ring as bioisostere for the C9—C10 double bond are described. The attempted synthesis of the cis-isomer leads mainly to unidentified products and the unexpected epimerization to the trans-derivative (VII). The latter is synthesized independently starting from the cyclopropane (I).