ChemInform Abstract: Hemisynthesis of Rhazinilam Analogues: Structure—Activity Relationships on Tubulin-Microtubule System.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
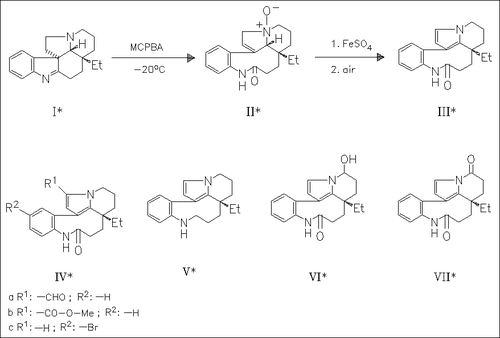
A series of rhazinilam analogues, e.g. (IV)—(VII), is prepared by conventional procedures to delineate some molecular features necessary for biological activity. However, none of these analogues have a higher activity than rhazinilam (III). In the course of this study, the formation of (III) from (I) is reexamined. The isolation of N-oxide (II) leads to a new proposal concerning the mechanism of this transformation (no yields given).